Why Does Water Move Up a Paper Towel? Capillary Action!
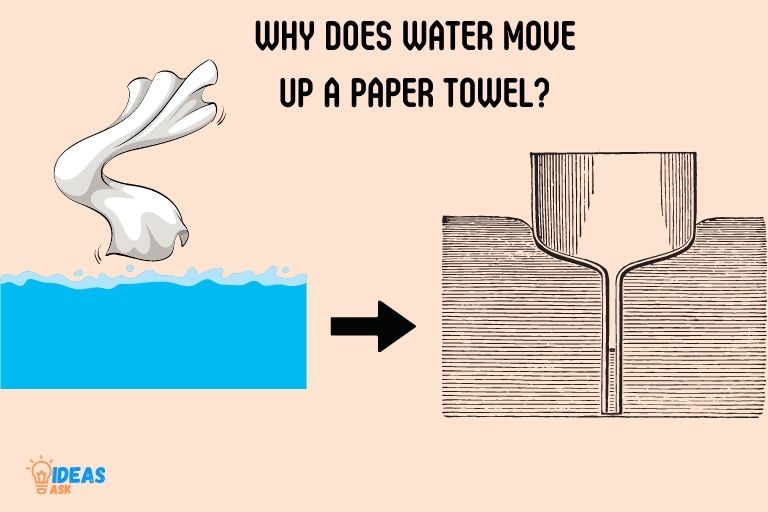
Water moves up a paper towel due to a phenomenon called capillary action, which is the result of cohesive and adhesive forces working together to push water into the tiny spaces between the fibers of the paper towel.
Capillary action occurs when the adhesive forces between water molecules and the fibers of the paper towel are stronger than the cohesive forces within the water itself.
This causes the water molecules to be attracted to the paper towel fibers, allowing them to move upwards against gravity.
Capillary action is an essential phenomenon in various aspects of nature and human-made devices. For instance, it aids in the transportation of water and nutrients in plants from their roots to their leaves.
Additionally, capillary action is crucial in ink pens as it allows ink to flow smoothly onto the writing surface.
Understanding and utilizing capillary action has allowed for the development of a wide range of applications in science, engineering, and everyday life.
5 Factors: Water Move Up a Paper Towel
| Factors | Explanation |
|---|---|
| Capillary Action | Water molecules are attracted to the paper towel fibers, causing the water to move upward against gravity. |
| Absorbency of Material | A paper towel is made of cellulose fibers, which can absorb and hold water, facilitating the upward movement. |
| Cohesion | Water molecules stick together, allowing the water to move as a mass through the paper towel fibers. |
| Adhesion | Water molecules adhere to the paper towel fibers, which helps in pulling water up the material. |
| Surface Tension | The surface tension of water molecules contributes to the capillary action, enabling the upward movement. |
Key Takeaway
Four Facts About the Capillary Action of Water in Paper Towels
Definition Of Capillary Action
Have you ever wondered why water moves up a paper towel? This phenomenon is known as capillary action, and it has a crucial role in ensuring the soil remains moist for plants to absorb water.
We will discuss the definition of capillary action, how it differs from other types of fluid transport, and its basic principle.
Explanation Of The Basic Principle
Capillary action is a result of the interplay between cohesive forces (attraction between molecules of the same type) and adhesive forces (attraction between unlike molecules).
The adhesive forces between water molecules and the paper towel are stronger than the cohesive forces of water resulting in a net upward force pulling the water upwards.
Moreover, the narrower the channel is, such as the small gaps within the fibers of a paper towel, the stronger the capillary action will be. This is because the adhesive force can push the liquid up further against gravity.
How It Differs From Other Types Of Fluid Transport
Capillary action is notably different from other types of fluid transport, such as diffusion or osmosis, as its movement is not driven by a concentration gradient.
Instead, it is associated with intermolecular attractive forces between the liquid and a solid that draws the liquid into narrow spaces.
Explanation Of The Concept Using Bullet Points
- Capillary action occurs when adhesive forces between unlike molecules are stronger than cohesive forces between same molecules.
- The narrower the channel, the stronger the capillary action.
- Capillary action moves liquid upwards regardless of any concentration gradient.
- Capillary action differs from diffusion and osmosis.
Understanding the concept of capillary action is crucial in various fields, notably in the pharmaceutical industry and water management. It also explains why water moves upward from the roots to the upper parts of a plant.
The Science Behind Capillary Action
This strange phenomenon is known as capillary action, which occurs due to the cohesive and adhesive forces between the paper towel fibers and water molecules.
Here’s a breakdown of the science behind capillary action.
Explanation Of The Role Of Adhesive Forces Between The Paper Towel Fibers And Water
Adhesion refers to the attractive force between molecules of different substances. In this case, the water molecules are attracted to the paper towel fibers, causing the water to spread out and “stick” to the towel.
The paper towel fibers are made up of tiny cellulose strands with small gaps in between them.
The water molecules are small enough to be drawn into these gaps, creating a capillary effect that pulls the water further up the towel.
Here are some key takeaways to remember about adhesive forces:
- Adhesion is the attractive force between molecules of different substances.
- The water molecules are attracted to the paper towel fibers, causing the water to spread out and “stick” to the towel.
- The tiny gaps in the paper towel fibers create a capillary effect that pulls the water further up the towel.
Explanation Of The Role Of Cohesive Forces Between Water Molecules
Cohesion refers to the attractive force between molecules of the same substance. In this case, the cohesive forces between water molecules create a “chain” of sorts, allowing them to stick together and move up the paper towel in unison.
This chain of water molecules is also responsible for surface tension, which is why small insects can walk on water without sinking.
Here are some key takeaways to remember about cohesive forces:
- Cohesion is the attractive force between molecules of the same substance.
- The cohesive forces between water molecules create a “chain” that allows them to stick together and move up the towel in unison.
- Cohesive forces are also responsible for surface tension.
Capillary action is a fascinating process that is due to the combined forces of adhesion and cohesion. By understanding this phenomenon, we can better understand how water moves through porous materials like a paper towel.
Factors Affecting Capillary Action
Capillary action refers to the ability of water (or any liquid) to move up a narrow space against gravity, drawing itself inward due to the forces of adhesion and cohesion.
For this reason, capillary action plays a significant role in many scientific and everyday applications, including paper towels!
In this section, we will delve into the key factors affecting capillary action, including pore size, fiber spacing, angle of contact, temperature, and atmospheric pressure.
Importance Of Pore Size And Spacing Between The Paper Fibers:
The size and spacing of pores between paper fibers significantly affect a paper towel’s capillary action.
Capillary action occurs when water adheres to the fibers of the material, which then create narrow channels where the water moves through.
Smaller pores and closer fiber spacing correspond to higher capillary action since the channels’ size is smaller, lessening the space that the water needs to travel.
- Smaller pore size and fiber spacing lead to quicker absorption and an enhanced capillary effect in paper towels.
- Larger pore size and more spacing between the fibers cause slower absorption and a weaker capillary effect.
The Relationship Between The Angle Of Contact And The Capillary Rise:
The angle of contact between the paper towel and the liquid (usually water) plays a crucial role in capillary action. It’s an essential factor in determining whether the liquid rises or falls in a small space.
- A smaller angle of contact affects the liquid surface tension and adhesion, causing more upward capillary action.
- A larger angle of contact, however, reduces the surface tension and adhesion between the liquid and paper towel, decreasing the capillary action.
The Effects Of Temperature And Atmospheric Pressure On Capillary Action:
Temperature and atmospheric pressure are external factors that influence the capillary action. As temperature increases and atmospheric pressure decreases, capillary action increases.
- Higher temperatures and lower atmospheric pressure lead to quicker absorption and enhanced capillary action in paper towels.
- Lower temperatures and higher atmospheric pressure cause slower absorption and weaker capillary action.
By understanding these factors affecting capillary action, we can see why water moves up a paper towel against the opposing force of gravity.
Proper understanding of these concepts and factors is essential in multiple scientific fields, including biomedicine and material science.
Demonstrations Of Capillary Action
Capillary action is a fascinating phenomenon that occurs when liquid flows through a porous material such as a paper towel.
The movement of water against gravity might seem counterintuitive, but capillary action is actually an essential component of our daily lives.
Understanding how this process works can help us make sense of the world around us and even inspire some fun experiments! We will explore demonstrations of capillary action and its practical applications.
Experiment To Showcase The Capillary Rise In A Paper Towel
Capillary action in a paper towel is easy to observe.
To showcase how it works follow these steps:
- Take one end of a paper towel and dip it into a glass of water.
- Hold the other end of the paper towel above the water, ensuring that it is hanging freely.
- Observe as the water begins to move through the paper towel and up towards the dry portion of the towel.
- Continued observation shows the water level gradually rising through the towel.
Other Practical Applications Of Capillary Action
Capillary action not only happens in our paper towels, but it plays a crucial role in various natural and human-made processes as well.
- Water transport in plants:
Capillary action helps plants absorb water and nutrients from the soil through their roots and distribute them to the rest of the plant.
- Ink pens:
Capillary action is also how ink is drawn into pens and delivered to the nib for writing.
- Scientific experiments:
In chemistry, capillary action is utilized in the chromatography of different liquids. This approach allows scientists to examine the components of various liquids.
- Medical testing:
Medical professionals use capillary action to screen for illnesses by analyzing blood and urine samples.
Capillary action is a fascinating aspect of our daily lives, and understanding how it works can provide us with insights into various natural phenomena.
We hope that by reading this article, you have gained a greater appreciation for the science behind capillary movements and its real-world applications.
FAQ On the Capillary Action of Water in Paper Towels
How Does Water Move Up A Paper Towel?
Water moves up a paper towel due to capillary action, which is the result of the paper towel’s small fibers.
What Is Capillary Action And How Does It Work?
Capillary action is the ability of a liquid to flow in narrow spaces without the assistance of external forces. It works by the combination of adhesive forces between the liquid and the surface of the paper towel, and cohesive forces within the liquid itself.
Why Do Paper Towels Absorb Water So Well?
Paper towels absorb water so well due to their high porosity, or the amount of empty space between fibers. This allows water to be absorbed quickly and efficiently.
Are All Paper Towels Equally Absorbent?
No, not all paper towels are equally absorbent. The absorbency of a paper towel depends on factors such as fiber composition, texture, and manufacturing process.
What Are Other Uses Of Capillary Action?
Capillary action is used in many practical applications, including in medical devices for blood testing, inkjet printers, and soil irrigation systems.
Conclusion
Ultimately, the phenomenon of water moving up a paper towel is due to a process known as capillary action. As liquids move through tiny spaces, such as the gaps between fibers in a paper towel, they can become attracted to the surrounding surfaces.
This attraction, or adhesion, causes the liquid to climb upwards. At the same time, molecules of the liquid also attract one another, creating cohesion. These two forces work together to pull the liquid up the fibers, against gravity. Understanding this process is not only fascinating but also has practical applications in everyday life.
From the absorption of spills to the functioning of plants, capillary action plays an important role in many natural and manmade processes. By delving deeper into the science behind this phenomenon, we can appreciate the intricate workings of the world around us.
So, the next time you reach for a paper towel, take a moment to marvel at the complex yet beautiful forces that allow it to do its job.